Safe and effective personal care and cosmetic products have the best market longevity. International regulatory agencies hold product manufacturers and distributors responsible for providing adequate safety information for all new products prior to market entry. These new-to-market products must be shown to not impart harm to human health when used under conditions of directed and foreseeable use. Products that may come into contact with the eyes—such as creams, serums, and cosmetics applied to the face as well as shampoos and conditioners—require ophthalmologist testing strategies to confirm that formulations do not irritate the eyes. To this end, an integrated, tiered testing strategy carried out by qualified, experienced professionals is recommended to create thorough safety documentation.
Tiered Approaches to Ophthalmological Testing
Formulation evaluation is necessary when introducing novel ingredients, ingredient vehicles, processing methods, or ingredient combinations and/or when making a specific safety claim (e.g., “Safe for Sensitive Eyes,” “Ophthalmologist Tested,” “Safe for Contact Lens Wearers,” etc.). Ingredients should be evaluated for safety in the final product concentration ranges, both individually and in when combined in the final product, to assess for the possibility of adverse interactions. Tiered approaches to ophthalmologist testing examine all available data to produce a well-rounded safety evaluation. Typically, tiered approaches require a toxicological profile and clinical assessments.
Toxicological Profiling
Data on ingredient physiochemical properties, such as chemical structure, stability and reactivity with other ingredients, should be evaluated in early phases of safety evaluation. These properties influence biological activity and determine whether acute and/or long-term toxicity occurs. Early profiling efforts should also include evaluation of historical in vivo toxicology data if available. Such screening outcomes are useful in early identification of likely ocular irritants and help to determine which battery of in vitro tests should be applied to formulation(s) under evaluation.
Novel formulations and/or processing methods need to be evaluated thoroughly. A combination of in vitro tests (which are approved by regulatory organizations such as ECVAM EURL) is ideal for providing additional confirmation of product safety prior to clinical trials. The precise battery of tests depends on the particular goals of the evaluation in question. For instance, if historical data suggests that an ingredient or formulation has a proclivity to cause membrane lysis or disturb macromolecules, in vitro and ex vivo tests designed to measure such outcomes are included in the overall in vitro/ex vivo testing battery.
Severe eye irritants may be evaluated by several regulatory agency-accepted in vitro and ex vivo ophthalmological tests, including but not limited to:
Hen’s Egg Test on the Chorioallantoic Membrane (HET-CAM) Assay
The HET-CAM Assay evaluates damage to the conjunctival membranes of the eye by measuring changes in the egg’s chorioallantoic membranes. Recommended for use in ophthalmological testing strategies by the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) in 2010, the ex vivo assay detects non-irritating, mild, and severe ocular irritants and may be adapted to ingredients with varied physiochemical properties.
The predicative capacity of each test should be considered when designing a hazard assessment workflow to meet required safety requirements. Generally, the structure of the assay dictates the degree of irritant detection. Confirmation of results depends on use of appropriate benchmarks, understanding assay limits and historic data on ingredients that have similar physicochemistry.
Clinical Evaluations
After initial toxicological profiling has determined ingredients and/or a formulation safe for human use, clinical trials with volunteer human subjects may be performed to test product compatibility in humans and support product efficacy claims. Human clinical trials lend additional data to confirm overall formulation safety and should be considered in the context of intended and foreseeable consumer use patterns (i.e., exposure area, application frequency, etc.).
Sample Ophthalmological Testing Workflow
Tiered integrative approaches should be devised based on regulatory agency-approved and industry-accepted assays (based on published literature and/or hazard expert experience) to provide a well-rounded hazard assessment.
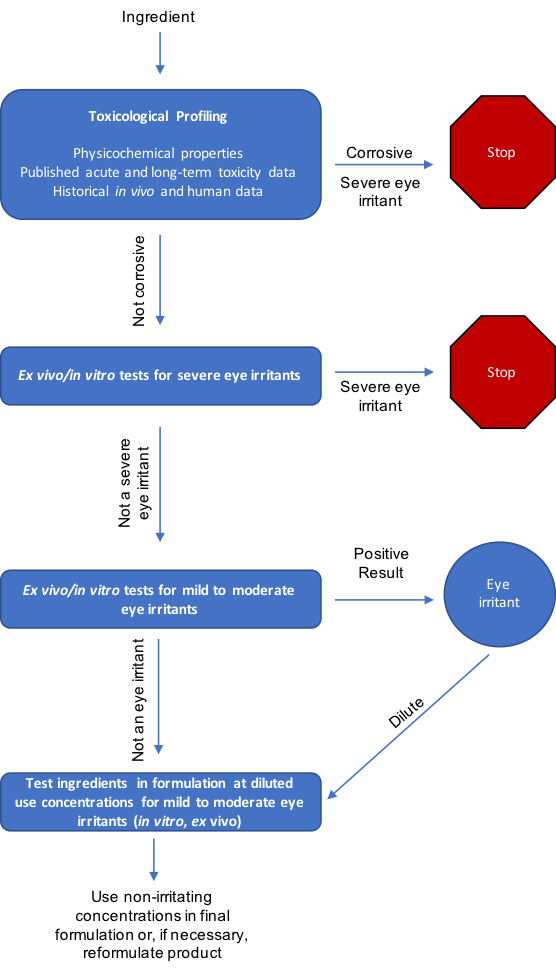 A sample ophthalmologist testing decision tree is outlined in Figure 1. Individual ingredients are first toxicologically profiled and tested to identify severe eye irritants. Severely irritating ingredients are either removed from the development pipeline or diluted to non-irritating concentrations. Non-irritant ingredients are ultimately tested in formulation(s) at use concentrations to confirm formulation safety for human use and identify likelihood of unforeseen injurious ingredient interactions. Here, testing multiple formulation versions (e.g., with varied ingredient concentrations) is useful to determine ideal final use concentrations. Additional safety confirmations in human clinical studies round out overall hazard safety assessments.
A sample ophthalmologist testing decision tree is outlined in Figure 1. Individual ingredients are first toxicologically profiled and tested to identify severe eye irritants. Severely irritating ingredients are either removed from the development pipeline or diluted to non-irritating concentrations. Non-irritant ingredients are ultimately tested in formulation(s) at use concentrations to confirm formulation safety for human use and identify likelihood of unforeseen injurious ingredient interactions. Here, testing multiple formulation versions (e.g., with varied ingredient concentrations) is useful to determine ideal final use concentrations. Additional safety confirmations in human clinical studies round out overall hazard safety assessments.
Summary
Product manufacturers and distributors are responsible for building satisfactory safety documentation on new products prior to market entry. For products that may come into contact with the eyes, an integrated, tiered testing strategy is recommended to confirm that such formulations do not irritate the eyes. Each ingredient in the proposed formulation should be evaluated individually and in the final formulation format using physiochemical and historical data combined with regulatory- and industry-approved assays to create thorough product safety documentation. Testing formulations in clinical studies is also recommended to support confidence in formulation safety for human use.


